A biomimetic blood vessel was fabricated using a modified 3D cell printing technique and bioinks, which were formulated from smooth muscle cells from a human aorta and endothelial cells from an umbilical vein. The result is a fully functional blood vessel with a dual-layer architecture that outperforms existing engineered tissue and brings 3D-printed blood vessels several fundamental steps closer to clinical use.
The engineered blood vessels were grafted as abdominal aortas into six rats. Over the next several weeks, scientists observed a transformation in which the rat’s fibroblasts formed a layer of connective tissue on the surface of the implant to integrate the fabricated vessel graft as part of the existing, living tissue. The results, published in Applied Physics Reviews, from AIP Publishing, include details on the triple-coaxial 3D printing technology they developed and their analysis of the unique architecture, physical strengths and biological activity of the engineered tissue.
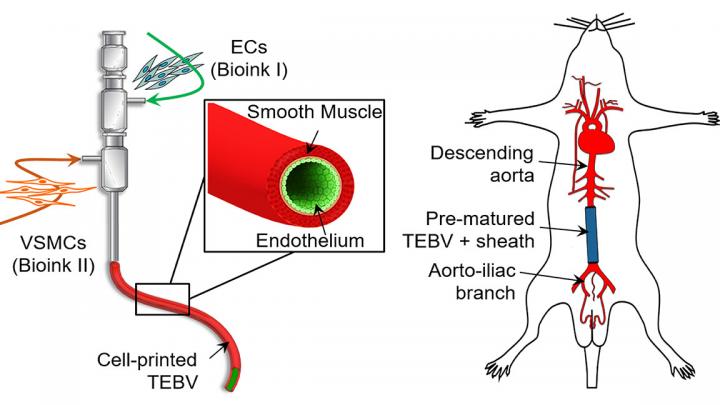
“The artificial blood vessel is an essential tool to save patients suffering from cardiovascular disease,” author Ge Gao said. “There are products in clinical use made from polymers, but they don’t have living cells and vascular functions. We wanted to tissue-engineer a living, functional blood vessel graft.”
Prior attempts to construct small-diameter blood vessels have yielded blood vessels that are fragile and prone to blockage. They often use a stripped-down version of extracellular material, such as collagen-based bioinks. In contrast, material from a native blood vessel contains collagen plus a collection of diverse biomolecules that provide a favorable microenvironment for vascular cell growth.
[rand_post]
Using these native-materials-based bioinks preserves the natural complexity of the blood vessel and accelerates the generation of functional vascular tissues, so they have enhanced strength and anti-thrombosis functions.
After fabrication, the printed blood vessel was matured in a lab that was designed to tune the vessel’s biological and physical characteristics to precise specifications of wall thickness, cellular alignment, burst pressure, tensile strength, and its ability to contract, mimicking natural blood vessel function.
The authors plan to continue to develop processes to increase the strength of the blood vessels closer to that of human coronary arteries. They also plan to perform long-term evaluation of vascular grafts, observing what happens as they continue to develop in place and become real tissue in the implanted environment.