Albert Einstein received the Nobel Prize for explaining the photoelectric effect: in its most intuitive form, a single atom is irradiated with light. According to Einstein, light consists of particles (photons) that transfer only quantised energy to the electron of the atom. If the photon’s energy is sufficient, it knocks the electrons out of the atom. But what happens to the photon’s momentum in this process? Physicists at Goethe University are now able to answer this question. To do so, they developed and constructed and new spectrometer with previously unattainable resolution.
Doctoral student Alexander Hartung became a father twice during the construction of the apparatus. The device, which is three meters long and 2.5 meters high, contains approximately as many parts as an automobile. It sits in the experiment hall of the Physics building on Riedberg Campus, surrounded by an opaque, black tent inside which is an extremely high performing laser. Its photons collide with individual argon atoms in the apparatus, and thereby remove one electron from each of the atoms. The momentum of these electrons at the time of their appearance is measured with extreme precision in a long tube of the apparatus.
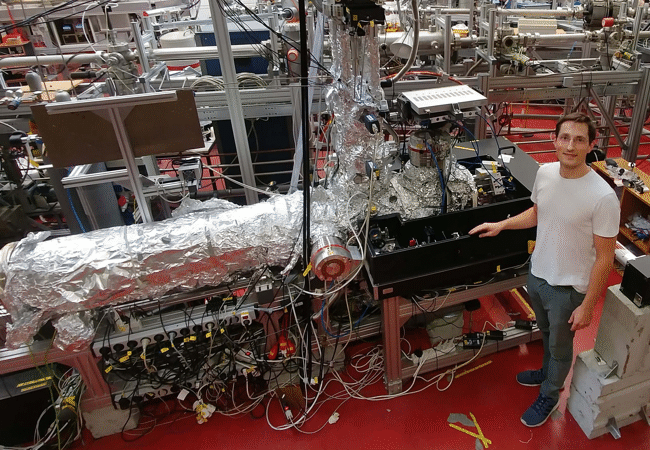
The device is a further development of the COLTRIMS principle that was invented in Frankfurt and has meanwhile spread across the world: it consists of ionising individual atoms, or breaking up molecules, and then precisely determining the momentum of the particles. However, the transfer of the photon momentum to electrons predicted by theoretic calculations is so tiny that it was previously not possible to measure it. And this is why Hartung built the “super COLTRIMS”.
[rand_post]
When numerous photons from a laser pulse bombard an argon atom, they ionise it. Breaking up the atom partially consumes the photon’s energy. The remaining energy is transferred to the released electron. The question of which reaction partner (electron or atom nucleus) conserves the momentum of the photon has occupied physicists for over 30 years. “The simplest idea is this: as long as the electron is attached to the nucleus, the momentum is transferred to the heavier particle, i.e., the atom nucleus. As soon as it breaks free, the photon momentum is transferred to the electron,” explains Hartung’s supervisor, Professor Reinhard Dörner from the Institute for Nuclear Physics. This would be analogous to wind transferring its momentum to the sail of a boat. As long as the sail is firmly attached, the wind’s momentum propels the boat forward. The instant the ropes tear, however, the wind’s momentum is transferred to the sail alone.
However, the answer that Alexander Hartung discovered through his experiment is – as is typical for quantum mechanics – more surprising. The electron not only receives the expected momentum, but additionally one third of the photon momentum that actually should have gone to the atom nucleus. The sail of the boat therefore “knows” of the impending accident before the cords tear and steals a bit of the boat’s momentum.
To explain the result more precisely, Hartung uses the concept of light as an electro-magnetic wave: “We know that the electrons tunnel through a small energy barrier. In doing so, they are pulled away from the nucleus by the strong electric field of the laser, while the magnetic field transfers this additional momentum to the electrons.”
Hartung used a clever measuring setup for the experiment. To ensure that the small additional momentum of the electron was not caused accidentally by an asymmetry in the apparatus, he had the laser pulse hit the gas from two sides: either from the right or the left, and then from both directions simultaneously, which was the biggest challenge for the measuring technique. This new method of precision measurement promises deeper understanding of the previously unexplored role of the magnetic components of laser light in atomic physics.