Supercapacitors, a type of electrical device that stores and releases energy, need a layer of electrolyte — an electrically conductive material that can be solid, liquid, or somewhere in between. Now, researchers at MIT and several other institutions have developed a novel class of liquids that may open up new possibilities for improving the efficiency and stability of such devices while reducing their flammability.
“This proof-of-concept work represents a new paradigm for electrochemical energy storage,” the researchers say in their paper describing the finding, which appears today in the journal Nature Materials.
For decades, researchers have been aware of a class of materials known as ionic liquids — essentially, liquid salts — but this team has now added to these liquids a compound that is similar to a surfactant, like those used to disperse oil spills. With the addition of this material, the ionic liquids “have very new and strange properties,” including becoming highly viscous, says MIT postdoc Xianwen Mao PhD ’14, the lead author of the paper.
“It’s hard to imagine that this viscous liquid could be used for energy storage,” Mao says, “but what we find is that once we raise the temperature, it can store more energy, and more than many other electrolytes.”
That’s not entirely surprising, he says, since with other ionic liquids, as temperature increases, “the viscosity decreases and the energy-storage capacity increases.” But in this case, although the viscosity stays higher than that of other known electrolytes, the capacity increases very quickly with increasing temperature. That ends up giving the material an overall energy density — a measure of its ability to store electricity in a given volume — that exceeds those of many conventional electrolytes, and with greater stability and safety.
[ad_336]
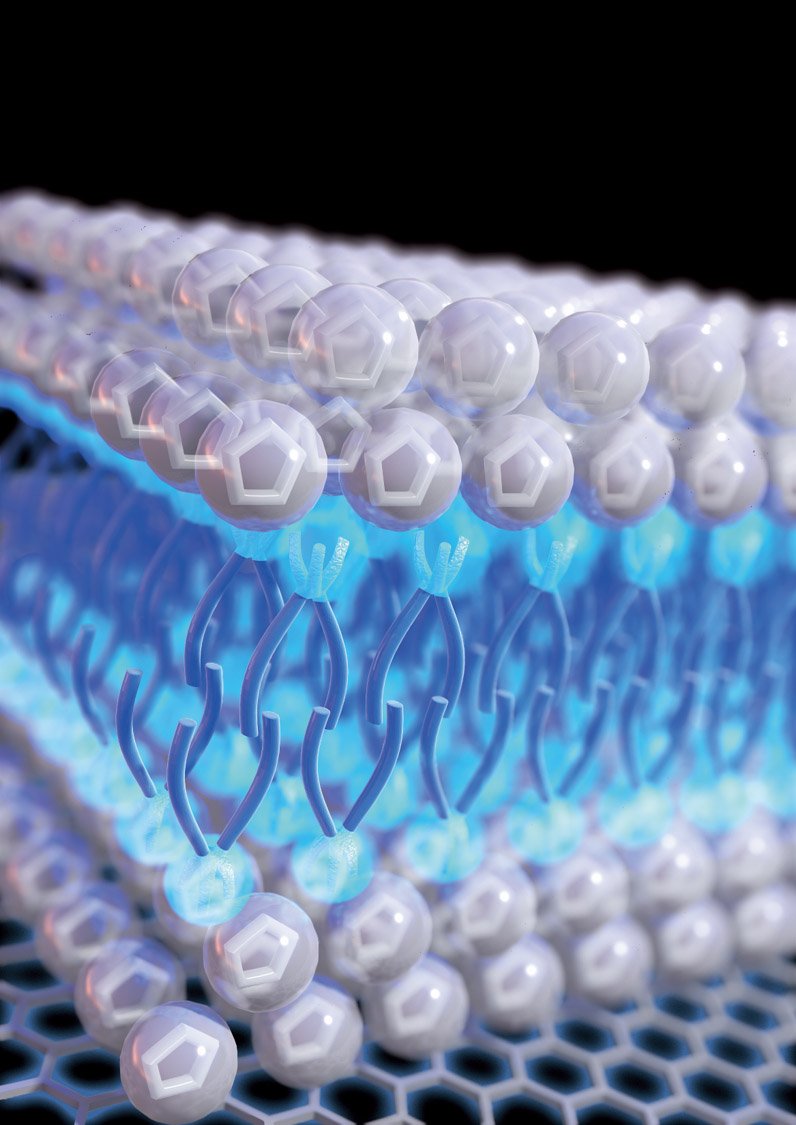
The key to its effectiveness is the way the molecules within the liquid automatically line themselves up, ending up in a layered configuration on the metal electrode surface. The molecules, which have a kind of tail on one end, line up with the heads facing outward toward the electrode or away from it, and the tails all cluster in the middle, forming a kind of sandwich. This is described as a self-assembled nanostructure.
“The reason why it’s behaving so differently” from conventional electrolytes is because of the way its molecules intrinsically assemble themselves into an ordered, layered structure where they come in contact with another material such as the electrode inside a supercapacitor device, says T. Alan Hatton, a professor of chemical engineering at MIT and the paper’s senior author. “It forms a very interesting sandwich-like double-layer structure.”
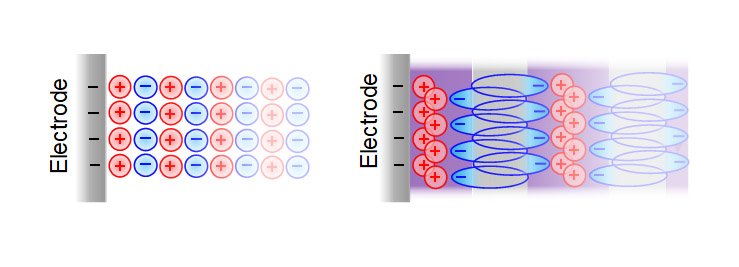
This highly ordered structure helps to prevent a phenomenon called “overscreening” that can occur with other ionic liquids, in which the first layer of ions at an electrode surface contains more countercharges than is on the surface. This can cause a more scattered distribution of ions, or a thicker ion multi-layer, and thus a loss of efficiency in energy storage, “whereas with our case, because of the way everything is structured, charges are concentrated within the surface layer,” Hatton says.
[rand_post]
The new class of materials, which the researchers call SAILs, for surface-active ionic liquids, could have a variety of applications for high-temperature energy storage, Mao says, for example for use in hot environments such as in oil drilling or in chemical plants. “Our electrolyte is very safe at high temperatures, and even performs better,” he says, unlike some electrolytes used in lithium-ion batteries that are quite flammable.
The material could really help to improve performance of supercapacitors, Mao says. Such devices can be used to store electrical charge and are sometimes used to supplement battery systems in electric vehicles to provide an extra boost of power. Using the new material instead of a conventional electrolyte in a supercapacitor could increase its energy density — the amount of energy that can be stored in a given volume of space — by a factor of four or five, Mao says. Using the new electrolyte, future supercapacitors may even be able to store more energy than batteries, potentially even replacing batteries in applications such as electrical vehicles, personal electronics, and even grid-level energy storage facilities.
[ad_336]
The material could also be useful for a variety of emerging separation processes, Mao says. “A lot of newly developed separation processes require electrical control,” in various chemical processing and refining applications and in carbon dioxide capture, for example, as well as resource recovery from waste streams. These ionic liquids, being highly conductive, could be well suited to many such applications, he says.
The material they initially developed is just an example of a variety of possible SAIL compounds. “The possibilities are almost unlimited,” Mao says. The team will continue to work on different variations and on optimizing its parameters for particular uses. “It might take a few months or years,” he says, “but working on a new class of materials is very exciting to do. There are many possibilities for further optimization.”
The research team included Paul Brown, Yinying Ren, Agilio Padua and Margarida Costa Gomes at MIT, Ctirad Cervinka at Ecole Normale Superieure de Lyon, in France, Gavin Hazell and Julian Eastoe at the University of Bristol, U.K., Hua Li and Rob Atkin at the University of Western Australia, and Isabelle Grillo at the Institut Max-von-Laue-Paul-Langevin in Grenoble, France. The work was supported by the MIT Energy Initiative, an MIT Skoltech fellowship and the Czech Science Foundation.