When studying how organisms evolve, biologists consider most traits, or features, as derived from some earlier version already present in their ancestors. Few traits are regarded as truly “novel.”
Insects were wingless, then winged. Animals were blind, then had eyes.
And in biology textbooks, novelty has a strict definition: it must have no relationship to any structure found in an ancestor and no relationship to any other body part elsewhere in the organism. By this definition, a dolphin’s pectoral fins are not a novelty because they are modified forelimbs that already existed.
However, some experts argue this creates a problem since it means novelty must seemingly arise from nothing. It must “pop up out of the blue” in evolutionary time.
Now evidence has emerged – in a study published Nov. 21 in the journal Science – that illuminates how new things can evolve. Moreover, this evidence has come from an unexpected source: the small, yet charismatic dung beetle.
“Dung beetles are fascinating creatures,” said Armin Moczek, the study’s senior author and a professor in the IU Bloomington College of Arts and Sciences’ Department of Biology.
[ad_336]
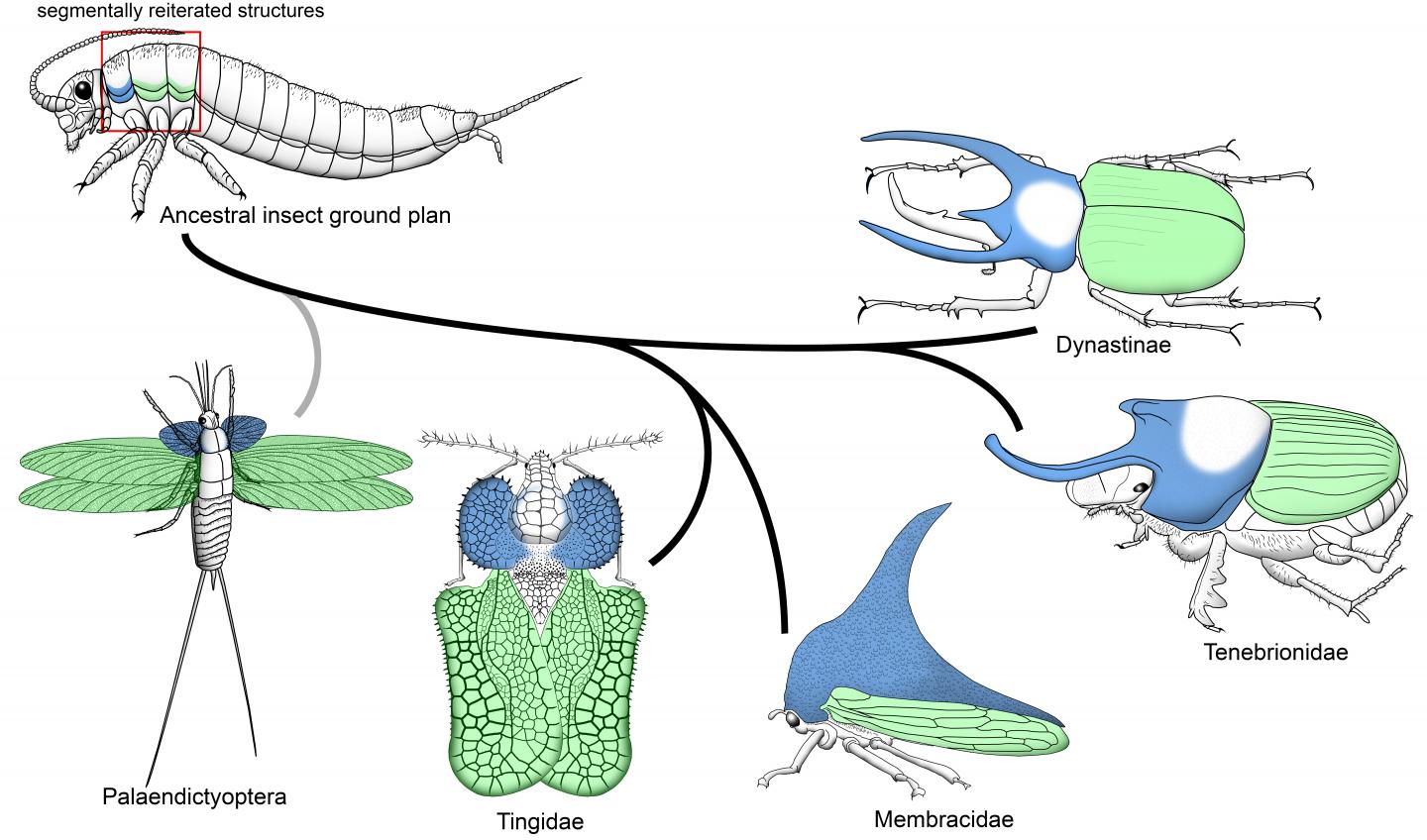
Among their many qualities, one structure has placed them at the forefront of discussions about novelty among researchers. That structure is their thoracic horn, which is regarded as a textbook example of an evolutionary novelty. This is because the thoracic horn is unique to horned beetles, and it appears to have no relationship to any other structure in the animal.
However, this view is now changing.

The new IU-led study, which is featured on the journal’s cover, provides evidence that the formation of the thoracic horn is instructed by the same core network of genes that led to the evolution of insect wings: flight structures that exist on neighboring thoracic segments. In fact, this ancient gene network predates not just wings and horns; it already existed before there were insects, and in every segment along the entire body.
“This work forces us to rethink what we mean by ‘novelty'”, Moczek said. “Each insect segment possesses this gene network, and as such, it is an ancient feature of their makeup. Yet, what each segment does with this network is so strikingly variable that it can yield traits that on the surface seem to have nothing in common, like wings and horns.”
To conduct the study, Moczek, along with lead authors Yonggang Hu and David M. Linz, knocked down various genes in this “wing gene network” in three different species of beetle: Onthophagus sagittarius, O. taurus, O. binodis. In all three species, the researchers were surprised to find that deactivation of genes in the network significantly affected not just the formation of wings, but also thoracic horns, causing them to be reduced or completely absent.
[rand_post]
They also showed that they could manipulate other genes and force the horns to transform into “ectopic” wings – or extra wings on the wrong part of the body – which provided additional evidence that thoracic horns and wings were interchangeable, alternative outputs of the same gene network.
This work also extends beyond just dung beetles and their thoracic horns.
“This body segment of insects, the first thoracic segment, is what we call a ‘hotspot’ of morphological innovation in insects,” said Moczek. “It harbors a lot of really diverse morphological features: horns in beetles, helmets in treehoppers, lateral projects of lace bugs, and many more. “This new evidence is profound since it suggests that all of this vast diversity, all these novelties, could in fact be enabled by a single gene network that was used millions of years ago to form the flight wings on other body segments.”

Hu, Linz and Moczek added that these results contribute to a growing call to re-examine the usefulness of defining morphological novelty as the absence of relatedness to other structures. Rather, this work suggests that novelty can emerge through such relatedness – and accordingly, textbook definitions of what constitutes novelty may need to be redefined.