Standard optical microscopes can image cells and bacteria but not their nanoscale features which are blurred by a physical effect called diffraction.
Optical microscopes have evolved over the last two decades to overcome this diffraction limit; however, these so-called super-resolution techniques typically require expensive and elaborated instrumentation or imaging procedures.
Now, Australian researchers from the ARC Centre of Excellence for Nanoscale BioPhotonics (CNBP) report in Nature Communications a simple way to bypass diffraction limitations using standard optical imaging tools.
Lead authors Dr Denitza Denkova, and Dr Martin Ploschner from the CNBP node at Macquarie University say, “Working closely with biologists has inspired us to look for a solution that can transform super-resolution from a complex and expensive imaging method into an everyday bio-imaging technique.”
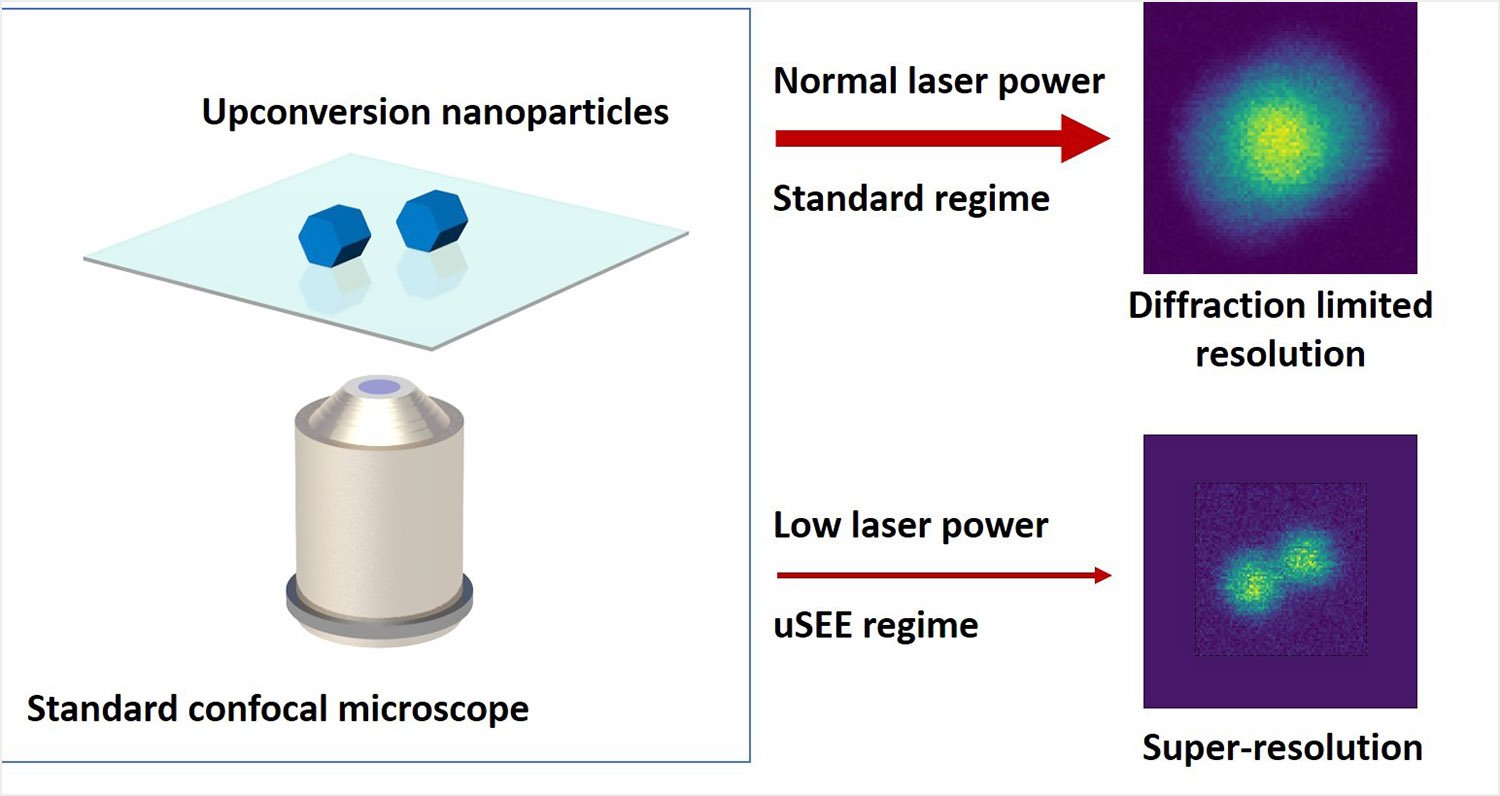
Dr Ploschner explains how the technique works: “We have identified a particular type of fluorescent markers, so-called upconversion nanoparticles, that can enter into a regime in which light emitted from the particles grows abruptly ¬- in a super-linear fashion – when increasing the excitation light intensity. Our key discovery is that if this effect is exploited under the right imaging conditions, any standard scanning optical microscope can spontaneously image with super-resolution.”
[ad_336]
“While we have chosen to demonstrate this upconversion super-linear excitation-emission (uSEE) on one of the most commonly used types of optical microscopes – a confocal microscope – practically any type of scanning microscope or microscope involving variations in the illumination intensity can benefit from this spontaneous improvement of the resolution.”
Dr Denitza Denkova says the uSEE approach improves the resolution beyond the diffraction limit simply by reducing the illumination intensity.
“Our approach works in the opposite direction to all other existing super-resolution methods; the lower the laser power, the better the resolution and the lower the risk of photo-damage to the bio-samples,” she says.
“Best of all, super-resolution can be achieved without setup modifications and image processing. Thus, this method has the potential to enter any biological lab, at practically no extra cost.”
“The value of our work is in realising the technique, for the first time, in a 3D biological setting, using biologically convenient particles. We suggest a modification of the composition of the nanoparticles and the imaging conditions, which triggers the spontaneous super-resolution to occur under a practically relevant microscopy configuration. We also develop a theoretical framework which allows end-users to adjust the particle composition and the imaging conditions and achieve super-resolution in their own laboratory setting.”
[rand_post]
“Our work enables microscopists to look in a new way with their existing tools.”
CNBP node leader at Macquarie University, Professor James Piper AM, who is also an author on the paper, says the concept has been around for a while, but its practical realisation was elusive due to the need to combine the distinct research fields of biology, material science, optical engineering and physics.
“CNBP offered an ideal meeting platform for scientists with diverse expertise to join forces and take the idea from the drawing board to a practical imaging tool,” Professor Piper says.